Batteries are devices that store and release electrical energy through chemical reactions. They are used in a wide range of applications, from powering handheld devices like smartphones and laptops, to providing backup power for homes and businesses, to fueling electric vehicles. Here is a basic overview of how batteries work:
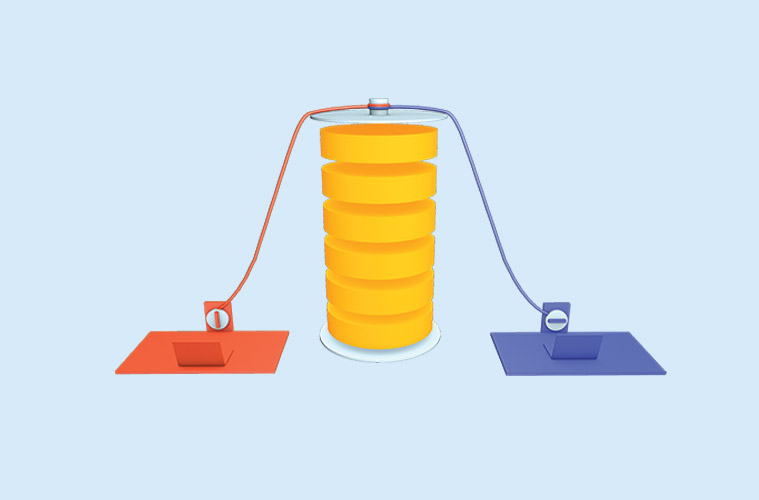
- Electrodes: Batteries have two electrodes, one positive (anode) and one negative (cathode), separated by an electrolyte.
- Chemical Reactions: When a battery is in use, a chemical reaction occurs at the electrodes, which generates an electrical current that can be used to power electrical devices. The direction of the current depends on the type of battery.
- Charging: Batteries can be recharged by reversing the chemical reaction, so that energy is stored in the battery instead of being used. The charging process typically requires an external source of energy, such as a solar panel or a charging station.
- Capacity: The amount of energy that a battery can store is measured in amp-hours (Ah) or milliampere-hours (mAh). The capacity of a battery is important because it determines how long the battery will last before it needs to be recharged.
- Types of Batteries: There are many different types of batteries, including lead-acid batteries, nickel-cadmium batteries, nickel-metal-Hydride batteries, and lithium-ion batteries. Each type of battery has different characteristics, such as energy density, cycle life, and charging times.
- Energy Efficiency: The energy efficiency of a battery refers to the proportion of energy that is stored in the battery that can be used to power electrical devices. Energy efficiency is an important factor to consider when choosing a battery because it affects the overall cost of using the battery.
In conclusion, batteries are essential devices that play a critical role in powering our modern world. Understanding the basics of how batteries work can help us make informed decisions when choosing the right battery for a particular application.